Abstract
Background
Inhaled corticosteroids (ICS) can help manage asthma exacerbations and symptoms in patients with severe asthma, but high doses have been associated with adverse effects and may not offer additional benefit to patients whose asthma is controlled by a biologic. Tezepelumab has been shown to reduce exacerbations and improve symptoms in patients with severe, uncontrolled asthma taking ICS.
Objective
To assess ICS dose reduction in patients with severe, uncontrolled asthma who received tezepelumab (210 mg every 4 weeks) versus placebo over 2 years.
Methods
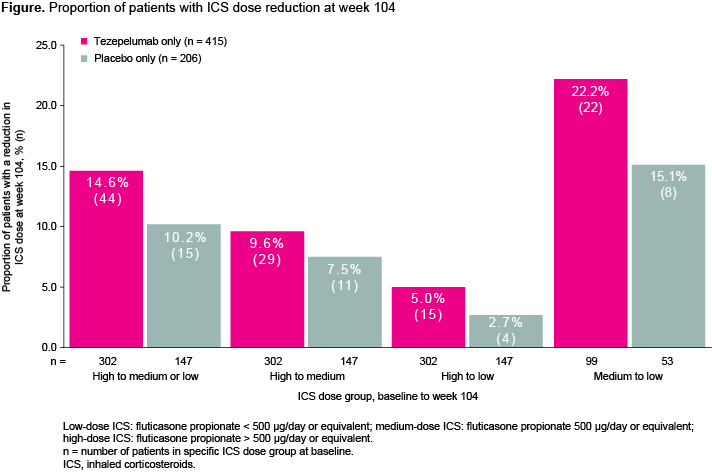
DESTINATION (NCT03706079) was a phase 3, multicentre, randomized, placebo-controlled, double-blind, extension study of patients (12?80 years old) who completed NAVIGATOR (NCT03347279) or SOURCE (NCT03406078). Patients included in this analysis were initially enrolled in the NAVIGATOR study and either received tezepelumab only or placebo only. All background medications (including ICS) were held constant in NAVIGATOR while step-down was allowed in DESTINATION. The proportion of patients who reduced their ICS dose at week 104 was assessed.
Results
Results are summarized in Figure 1.
Conclusion
Following a constant ICS dose in year 1, tezepelumab treatment led to numerically more patients achieving ICS dose reduction in year 2 versus placebo in patients with severe, uncontrolled asthma.