Abstract
Background
Tezepelumab reduced the annualized asthma exacerbation rate in adolescents with severe, uncontrolled asthma in the phase 3 NAVIGATOR (NCT03347279) and the phase 3 long-term extension DESTINATION (NCT03706079) studies.
Objective
To more completely describe the safety and efficacy of tezepelumab (210 mg every 4 weeks) in adolescents (?12?<18 years) over 2 years.
Methods
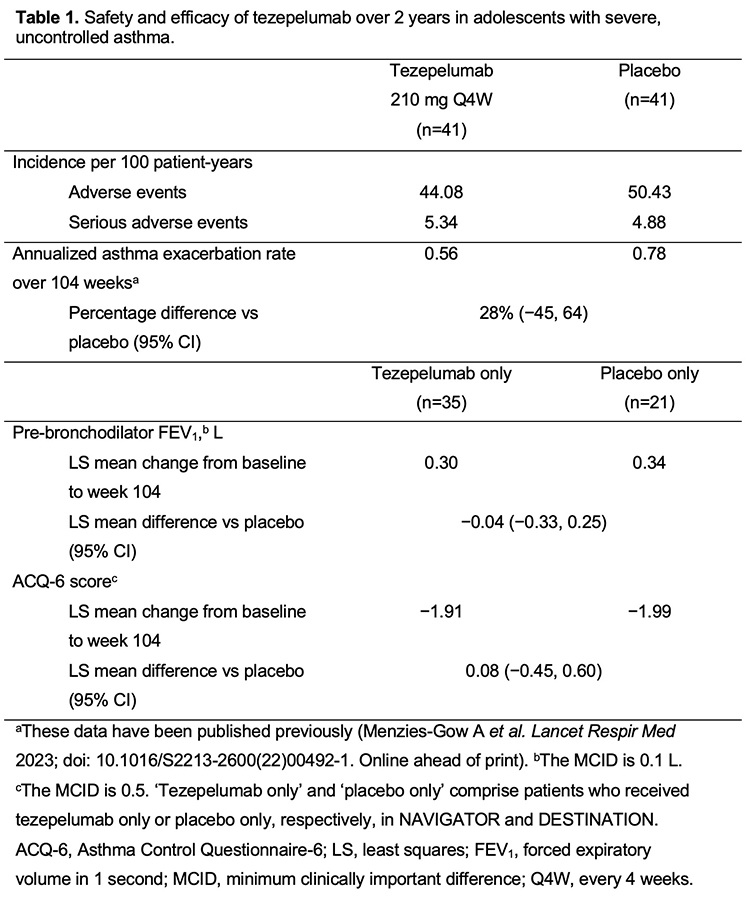
Patients (12?80 years old) who completed NAVIGATOR could enrol in DESTINATION, a multicentre, randomized, placebo-controlled, double-blind, extension study. Patients previously randomized to tezepelumab continued treatment with tezepelumab. Those previously randomized to placebo were re-randomized 1:1 to placebo or tezepelumab. Exposure-adjusted incidence rates of adverse events and serious adverse events and the annualized asthma exacerbation rate were assessed over 104 weeks in adolescents who received ?1 dose of tezepelumab or placebo. Pre-bronchodilator forced expiratory volume in 1 second and Asthma Control Questionnaire-6 score were assessed over 104 weeks in adolescents who received tezepelumab only or placebo only.
Results
Results are summarized in Table 1.
Conclusion
In addition to reducing asthma exacerbations, tezepelumab was well tolerated for 2 years and improved lung function and asthma control from baseline in adolescents with severe, uncontrolled asthma.