Abstract
Background
Asthma remission is characterized by long-term disease stabilization and control with or without ongoing treatment.
Objective
This post hoc exploratory analysis assessed the proportion of patients who received tezepelumab in DESTINATION (NCT03706079) who achieved on-treatment remission over 2 years.
Methods
DESTINATION was a phase 3, multicentre, randomized, placebo-controlled, double-blind extension study. Patients (12?80 years old) included in this analysis received tezepelumab in both NAVIGATOR (NCT03347279) and DESTINATION (tezepelumab only) or placebo in both NAVIGATOR and DESTINATION (placebo only). Patients received treatment up to week 104. Remission was predefined as an Asthma Control Questionnaire-6 score ? 1.5, stable lung function (a forced expiratory volume in 1 second > 95% of baseline) at the end of each year, and no exacerbations or use of oral corticosteroids during the time periods assessed.
Results
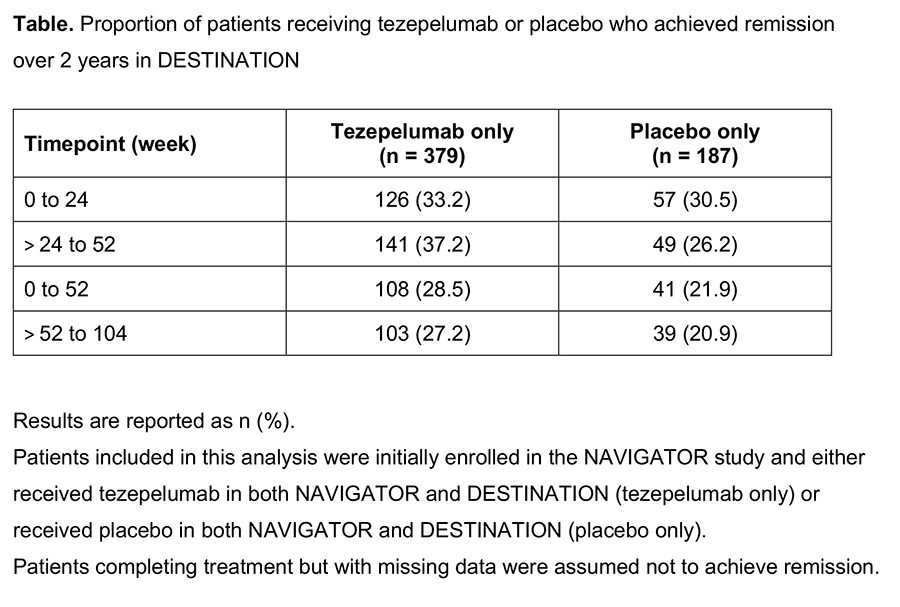
The proportion of patients who achieved remission in the tezepelumab only and placebo only groups are summarized in the Table. An intercurrent exacerbation requiring systemic corticosteroid use was the main reason why a patient no longer met the definition of remission.
Conclusion
Among patients with severe, uncontrolled asthma, a numerically greater proportion of patients who received tezepelumab than placebo achieved remission during the time periods assessed.