Abstract
Endoscopic lung volume reduction (ELVR) with valves is an effective treatment in patients with severe lung emphysema. It has been shown to improve both lung function and quality of life. But patients with a very low FEV1 (FEV1?20%) are often excluded from ELVR. Using real-world data from the LE-R we compared outcomes and safety regarding baseline FEV1 up to 6 months.
All data originates from the LE-R, a prospective multicenter clinical study, focused on treating lung emphysema patients. Two groups were created: FEV1?20% and FEV1 20-45%. Data were collected at baseline and after 6 months regarding pulmonary function, exercise capacity (6-MWD), quality of life (SGRQ) and serious adverse events (SAE).
298 cases were analyzed. Table 1 shows the comparison from baseline to the 6-month follow-up in both groups. In the first 90 days pneumothorax was the most common SAE (FEV1?20%: 7.7% vs. FEV1 20-45%: 22.1%; p=0.624). After 90 days acute exacerbation of COPD was the most observed SAE (FEV1?20%: 17.6% vs. FEV1 20-45%: 4.4%; p=0.065). There were no deaths in the FEV1?20% group.
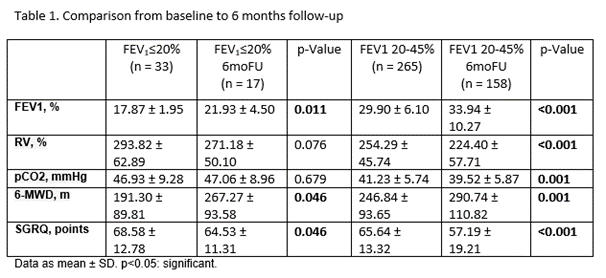
The data shows that ELVR can be an effective and safe treatment up to 6 months, in a highly selected group of patients with a very low FEV1.