Abstract
Introduction/Aim: RSV can cause severe respiratory disease in older adults with cardiorespiratory conditions. In an ongoing phase 3 placebo-controlled study (NCT04886596), the vaccine efficacy (VE) of RSVPreF3 OA during the first RSV season was 82.6% against RSV-related lower respiratory tract disease (RSV-LRTD) and 71.7% against RSV-related acute respiratory illness (RSV-ARI) in ?60-year-olds. We present VE in participants with co-existing cardiorespiratory conditions of interest associated with a higher risk of severe RSV disease outcomes.
Methods: Adults ?60 years were randomized 1:1 and received 1 dose of RSVPreF3 OA or placebo. VE against first RSV-LRTD and RSV-ARI episodes was assessed in subgroups of specific interest.
Results: Of the 12,467 RSVPreF3 OA and 12,499 placebo recipients, 20.0% and 19.4%, respectively, had ?1 cardiorespiratory condition of interest. Incidence rates of RSV-LRTD and RSV-ARI were higher in placebo recipients with ?1 cardiorespiratory condition of interest vs those with no medical conditions of interest. VE was 92.1% (RSV-LRTD) and 88.1% (RSV-ARI) (Figure). 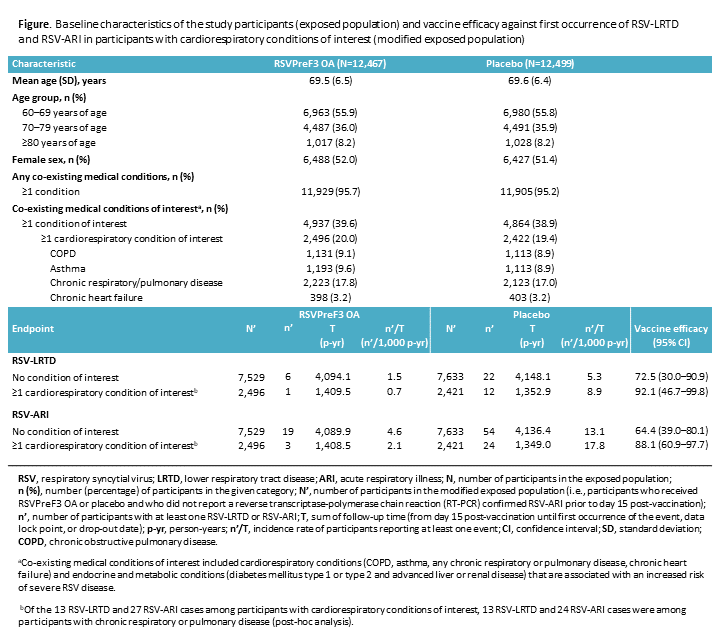
Conclusion: RSVPreF3 OA is efficacious against RSV-LRTD and RSV-ARI in ?60-year-olds with cardiorespiratory conditions of interest, a population who may benefit the most from protection against RSV.
Funding: GlaxoSmithKline Biologicals SA