Abstract
Introduction
Clinical trial evidence shows that biologic efficacy for severe asthma is related to biomarkers, but the extent to which this can be used to select treatments in the real-world is unclear.
Aims and Objectives
We aimed to determine if pre-biologic measurements of biomarkers were predictive of lung function
and asthma control in severe asthma patients treated with anti-IL5/5R or anti-IgE biologics in real-
world settings.
Methods
The International Severe Asthma Registry collects data from 23 countries. We included all patients
aged ?18 years with data on blood eosinophil count (BEC), fractional exhaled nitric oxide (FeNO) or
serum immunoglobulin-E (IgE) and with pre- and post-biologic FEV 1 and asthma control.
Associations between outcomes one year after biologic initiation and highest pre-biologic biomarker
levels were examined using regression models, adjusting for baseline measurement of the relevant
outcome.
Results
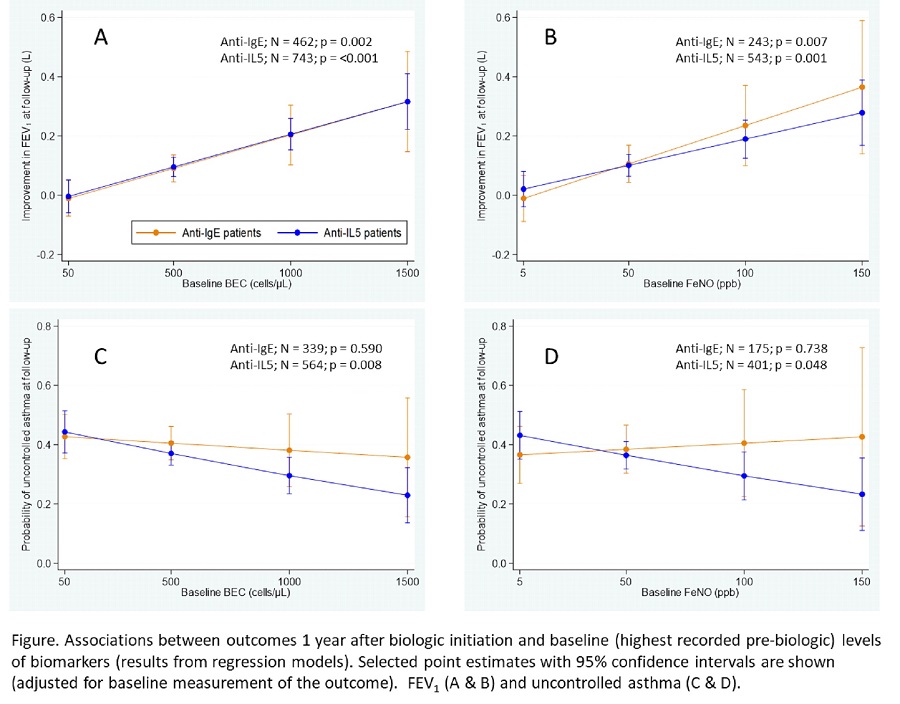
Higher baseline BEC and FeNO were associated with greater post-treatment FEV 1 improvement in
anti-IgE and anti-IL5/5R patients, and reduced odds of uncontrolled asthma in anti-IL5/5R patients
(Figure). IgE level was not associated with either of these outcomes. A combination of BEC and FeNO
predicted follow-up FEV 1 improvement more accurately than either alone (p<0.01).
Conclusions
The results support the use of BEC and FeNO to help to identify patients who will benefit most from
biologics.