Abstract
Rationale: The association of bronchiectasis (BE) with severe asthma with eosinophilic phenotype (SEA) is quite frequent. BE is a complex disease that coexists with other respiratory conditions. As comorbidities can influence or even predict treatment effect, we aimed to examine the effect of mepolizumab in patients with or without BE in the REDES study.
Methods: The observational multicenter REDES study assessed the effectiveness of mepolizumab 100mg SC every 4 weeks in 318 SEA patients in Spain for 12 months. This is a post-hoc analysis of these results stratified according to the presence or absence of concomitant bronchiectasis.
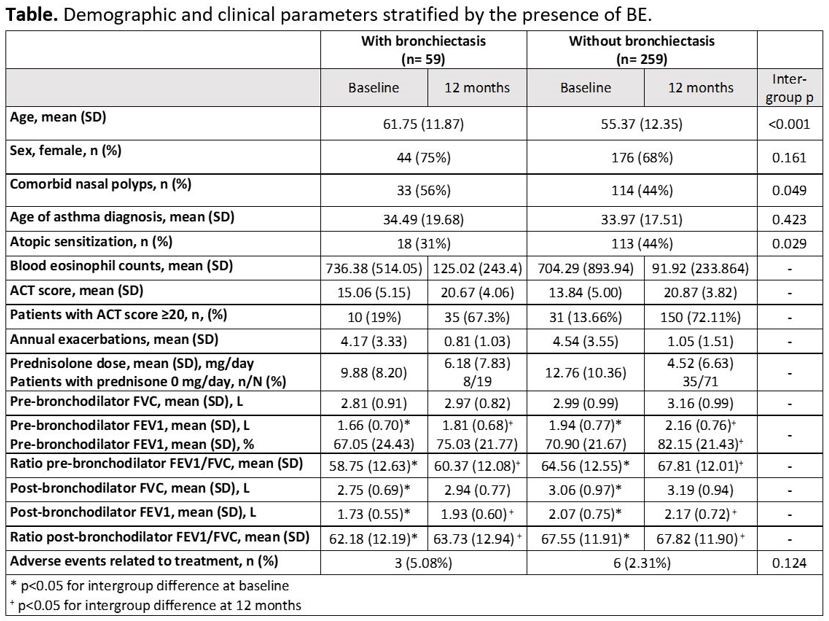
Results: 59 patients (19%) had concomitant BE. These patients were older (62 yrs vs 55 yrs), less atopic (31% vs 44%), and had comorbid nasal polyps more frequently (56% vs 44%) than patients without BE. There were significant baseline differences in lung function between subgroups, probably due to the difference in age as well as the presence of BE. The improvements in all clinical parameters were similar in both groups and there were no differences in the number and nature of adverse events between them (Table).
Conclusions: Severe asthma with concomitant BE is a common but not so well-known phenotype of severe asthma patients. These results reinforce, in a multicenter study, the effectiveness and safety of mepolizumab in this type of patients.
Funding: GSK (GSK ID 213172)