Abstract
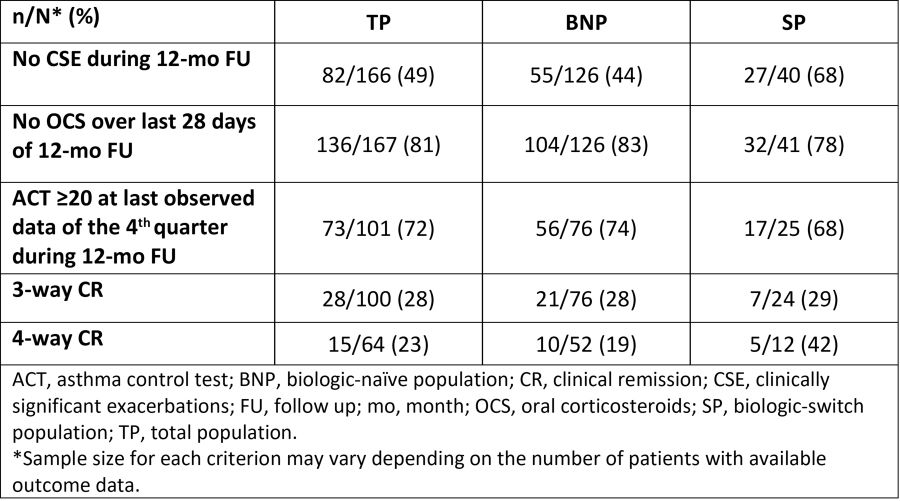
The retrospective, observational, self-controlled REMIT study enrolled 170 pts with SEA who started mepolizumab 100 mg SC Q4W, from 17 Taiwanese centres with 12-month (mo) follow-up (FU). This post-hoc analysis of 167 pts (excluding 3 pts who died during FU) assessed the proportion of pts achieving CR after mepolizumab use in the total (TP), biologic-naïve (BNP; TP excluding pts switching from omalizumab) and biologic-switch (SP; TP who switched from omalizumab) populations. Criteria for 3-way composite CR were: no clinically significant exacerbations (CSE; no oral corticosteroids [OCS], ER visits, or hospitalisation) during 12-mo FU, no OCS over the last 28 days of 12-mo FU, and ACT score ?20 at the last observed data of the 4th quarter of 12-mo FU. 4-way CR also included stabilisation in lung function (?100 ml decrease from baseline) during 12-mo FU. 28% TP, 28% BNP and 29% SP pts achieved 3-way CR (N=100); 23% TP, 19% BNP and 42% SP achieved 4-way CR (N=64) (Table). When analysing individual components of CR, 49% TP, 44% BNP and 68% SP pts had no CSE at 12 mo; 81% TP, 83% BNP and 78% SP pts had no OCS use over the last 28 days of 12-mo FU, and ACT score was ?20 in 72% of the TP, 74% BNP and 68% SP. Overall, mepolizumab effectively achieved multi-component CR in Chinese pts with SEA, including pts who switched from other biologics.
Funding: GSK (214665)