Abstract
Background
Results of clinical trials may not reflect the benefits of asthma biologics in real-world settings.
Objective
To describe clinical remission following initiation of biologics in adults with severe asthma in ISAR.
Methods
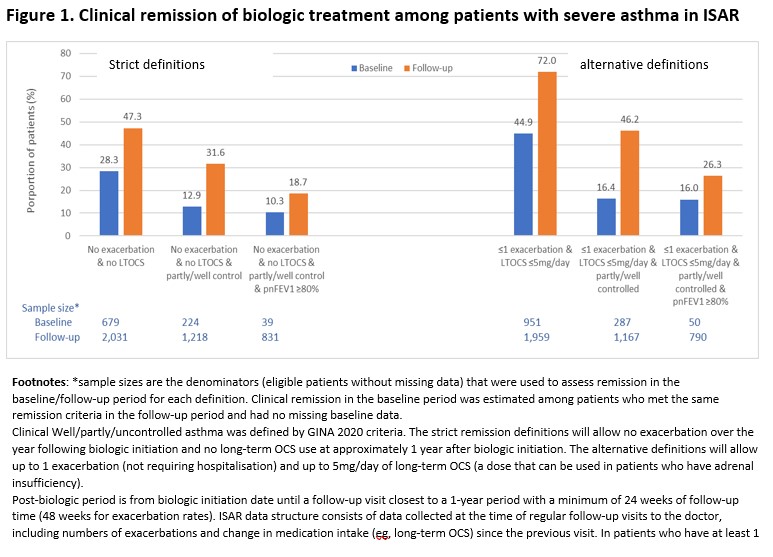
This multi-country (n=23), registry-based study included patients ?18 years who initiated treatment with biologics (anti-IL5/5R, anti-IgE, anti-IL4/IL13). Remission during the 1 year (min. 6 months) post-initiation was defined as a composite of 2, 3, or 4 endpoints: no exacerbations, no long-term OCS (LTOCS) use, partly/well controlled asthma, and predicted normal (p.n.FEV1) ?80%. Alternative definitions of remission using ?1 exacerbation and ?5 mg/day LTOCS were also explored.
Results
Of 3348 eligible adults, median age was 54 years, 61.6% were female. At baseline vs post-initiation, 23.3% vs 54.7% had no exacerbation, 66.7% vs 79.3% did not require LTOCS, 9.3% vs 32.7% had well-controlled asthma, and 43.0% vs 46.4% had p.n.FEV1?80%. Remission in 4 endpoints was achieved in 18.7% and 26.3% patients using the strict and alternative definitions (Figure 1).
Conclusion
About one-in-five adults with severe asthma met criteria for clinical remission in all 4 endpoints following biologic initiation. The findings suggest that biologics can achieve remission in the real-world, however, medical unmet need remains among some patients with the current treatment paradigm.