Abstract
Background: Improving lung function is key to managing paediatric asthma. Dupilumab (DPL), a fully human monoclonal antibody, blocks the shared receptor component of IL?4/IL-13, key drivers of type 2 asthma. Long-term DPL use in EXCURSION (NCT03560466) sustained lung function improvement in 6?11-year-old patients (pts) with uncontrolled type 2 asthma who had completed VOYAGE (NCT02948959); rapid improvement in lung function was observed in patients who switched from placebo (PBO) to DPL.
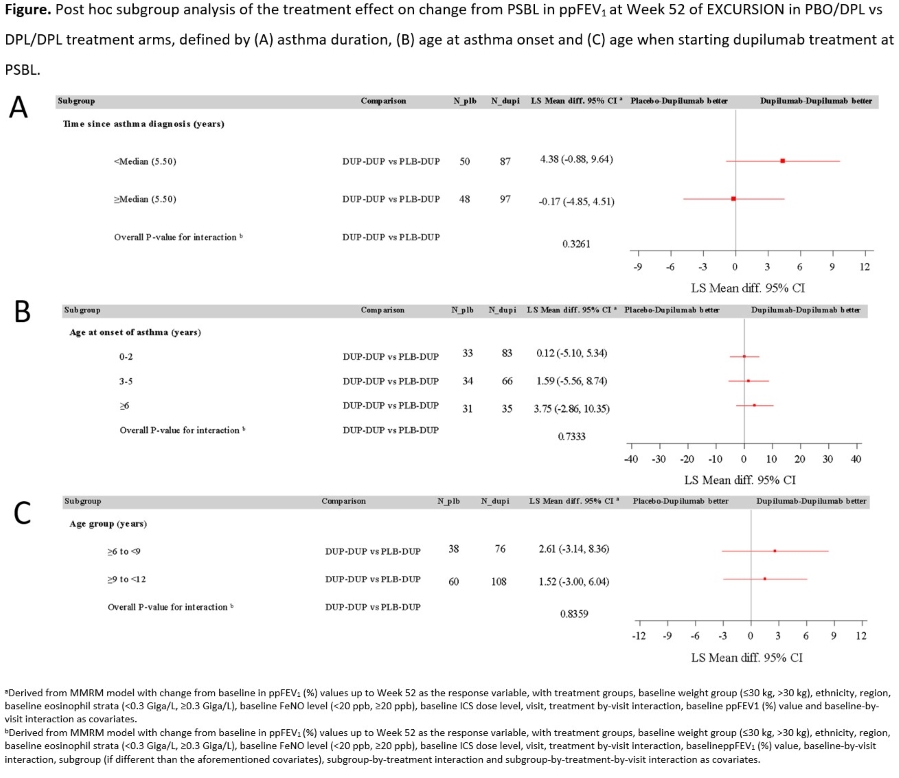
Aim: To analyse numerically higher lung function improvements seen consistently in the DPL/DPL vs PBO/DPL arms in EXCURSION..
Methods: Pts received add-on DPL 100/200 mg or PBO every 2 weeks (q2w; Wks) for 52 Wks in VOYAGE and DPL 100/200 mg q2w for 52 Wks in EXCURSION. Endpoint: change from parent study (PS) baseline (BL) in percent predicted (pp) FEV1 by asthma duration and age at onset or DPL start at PSBL.
Results: At Wk 0, 106 pts were included in PBO/DPL arm and 209 in DPL/DPL arm. At Wk 52, change from BL in ppFEV1 in DPL/DPL vs PBO/DPL was numerically greater (Figure) with shorter asthma duration (interaction p-value P=0.33), older age at onset (P=0.73) and younger age at DPL start (P=0.84); none of these reached statistical significance.
Conclusion: Improved lung function response to DPL over 1-2 years of treatment in 6?11-year-old pts with uncontrolled type 2 asthma did not differ by asthma duration or age at onset or DPL start.