Abstract
Background: There is a need to decrease recurrent rescue systemic corticosteroid (SCS) exposure for the treatment of exacerbations in patients with asthma, especially children. Dupilumab (DPL) is a fully human mAb that blocks the shared receptor component of IL?4/13, key and central drivers of type 2 (T2) inflammation in multiple diseases. In VOYAGE (NCT02948959), DPL was generally well tolerated, significantly reduced severe asthma exacerbations and improved lung function, in children with uncontrolled moderate-to-severe asthma.
Objective: This post hoc analysis of VOYAGE assessed DPL efficacy in reducing the need for rescue SCS in children.
Method: Children aged 6?11 years were randomized to DPL 100/200mg or placebo (PBO), every 2 weeks for 52 weeks. The annualized number of total SCS courses (including oral, IM, IV) was analysed in subgroups of patients stratified by number of exacerbations prior to VOYAGE.
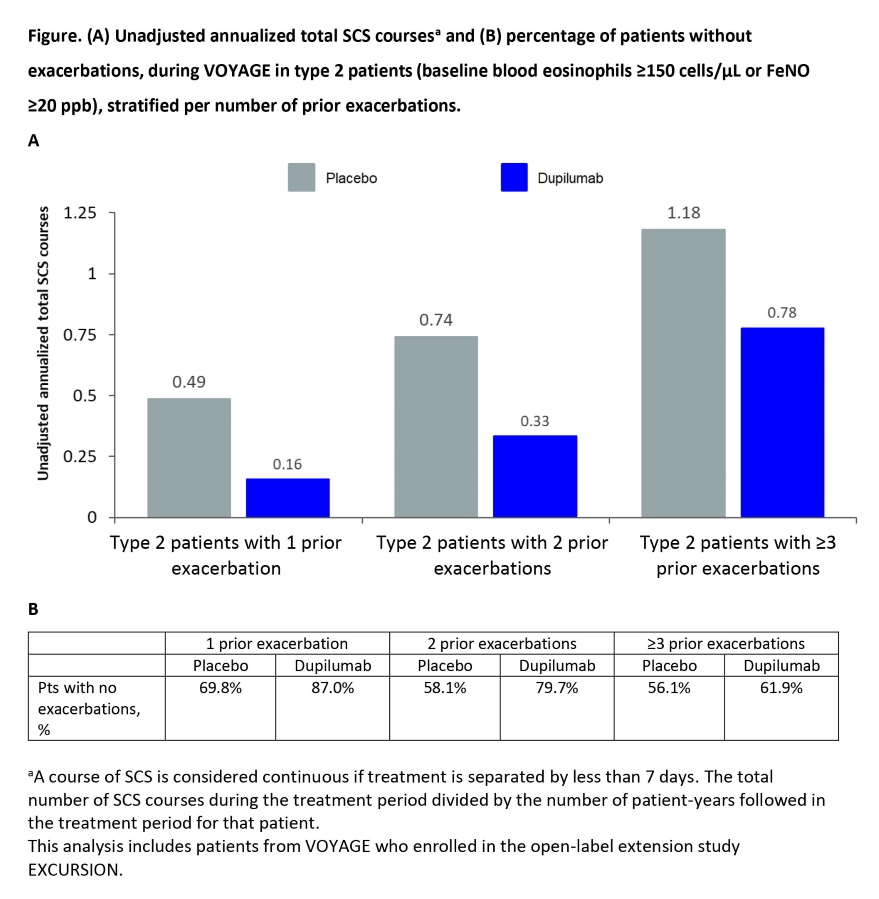
Results: Unadjusted annualized total SCS courses in T2 patients with 1, 2 and ?3 prior exacerbations were 0.49 (PBO, n=43) vs 0.16 (DPL, n=77), 0.74 (PBO, n=31) vs 0.33 (DPL, n=69), and 1.18 (PBO, n=32) vs 0.78 (DPL, n=63), respectively (FigA). DPL treatment led to fewer exacerbations (FigB).
Conclusion: In children with uncontrolled moderate-to-severe asthma, DPL was effective in reducing SCS burden related to exacerbations, regardless of prior exacerbation history.