Abstract
Background: Co-administration of oral phosphodiesterase 5 inhibitors (PDE5i) with oral guanylate cyclase stimulators (sGCs) are contraindicated due to systemic side effects. MK-5475, an inhaled sGC, is being developed for pulmonary hypertension and may allow combined use with oral PDE5i.
Methods: To evaluate a potential interaction between inhaled MK-5475 and oral sildenafil, 19 healthy participants (age: 45-65 yrs) were randomized (1:1) to 2 treatment sequences consisting of 2 periods with 36-hr washout. Treatment involved 3 days dosing with open-label oral sildenafil (20 mg 3x per day [TID]) and single-inhaled dose of double-blind MK-5475 240 µg (dry powder inhaler) or placebo on Day 3. Primary endpoints: safety assessed by adverse events (AEs) and change from baseline in SBP, DBP and HR on Day 3 over 5 hrs. Primary hypothesis was supported if 90% CI for minimum SBP lies above -6 mmHg.
Results: Incidences of AEs were comparable across groups with no serious AEs, discontinuations due to AEs or deaths. MK-5475 did not meaningfully change systemic hemodynamics (Table).
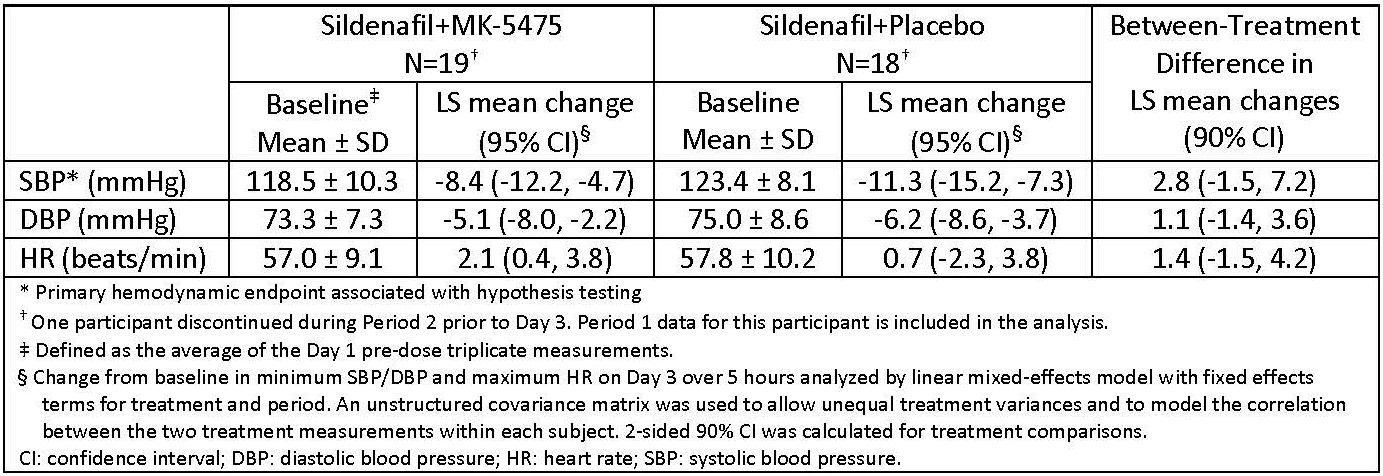
Conclusions: Inhaled MK-5475 was well tolerated when administered on top of background sildenafil 20 mg TID, without effects on systemic hemodynamics versus placebo. The lack of additive systemic effects on blood pressure further documents pulmonary selectivity of inhaled MK-5475 including when co-administered with sildenafil.