Abstract
Background
Registry data show an increasing number of elderly patients with pulmonary arterial hypertension (PAH) with cardiovascular comorbidities. Effects of these comorbidities on response to PAH treatment is not well understood and guidelines do not provide evidence-based treatment recommendations in this population.
Objective
To explore the effects of inhaled treprostinil (iTre) on PAH patients with ?1 cardiovascular comorbidity in the pivotal TRIUMPH study.
Methods
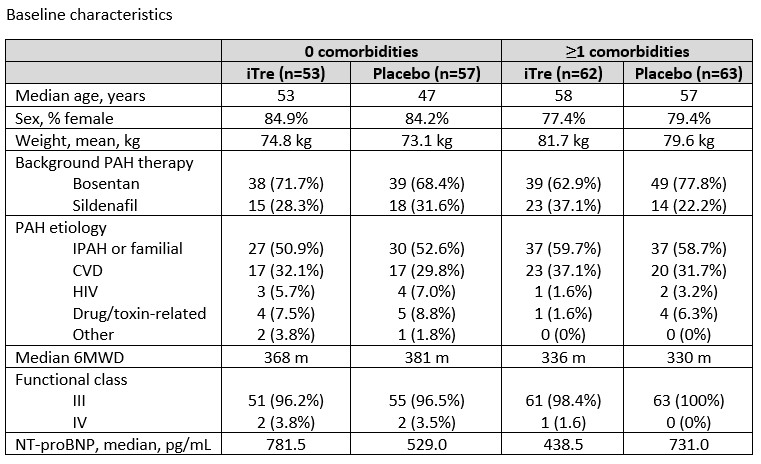
Patients (n=235) were classified as either having 0 or ?1 cardiovascular comorbidities. Median difference in 6MWD change from baseline was determined by the Hodges-Lehman estimate and the Wilcoxon rank sum test. No imputation was used.
Results
There were 110 and 125 patients with 0 and ?1 comorbidities respectively. At Baseline, patients with comorbidities weighed more, had a lower 6MWD, and more were male compared to patients without comorbidities. In patients with 0 comorbidities, iTre had a placebo-corrected change from baseline in 6MWD of 27 m (p=0.0057). In patients with ?1 comorbidities, the placebo-corrected change from baseline in 6MWD was 20 m (p=0.027).
Conclusions
In this post-hoc analysis of the TRIUMPH study, PAH patients without comorbidities had a greater 6MWD improvement than those with comorbidities. Inhaled treprostinil significantly improved exercise capacity versus placebo, irrespective of the presence of comorbidities.