Abstract
AIMS AND OBJECTIVES: The IMPACT trial showed greater treatment effects for use of inhaled corticosteroids in patients with COPD with increased blood EOS count (Pascoe, S. et al. Lancet Respir Med 2019;7:745-56). We assessed long-term incremental cost-effectiveness of FF/UMEC/VI vs FF/VI and UMEC/VI by baseline blood EOS count, from a UK National Health Service perspective, using IMPACT data.
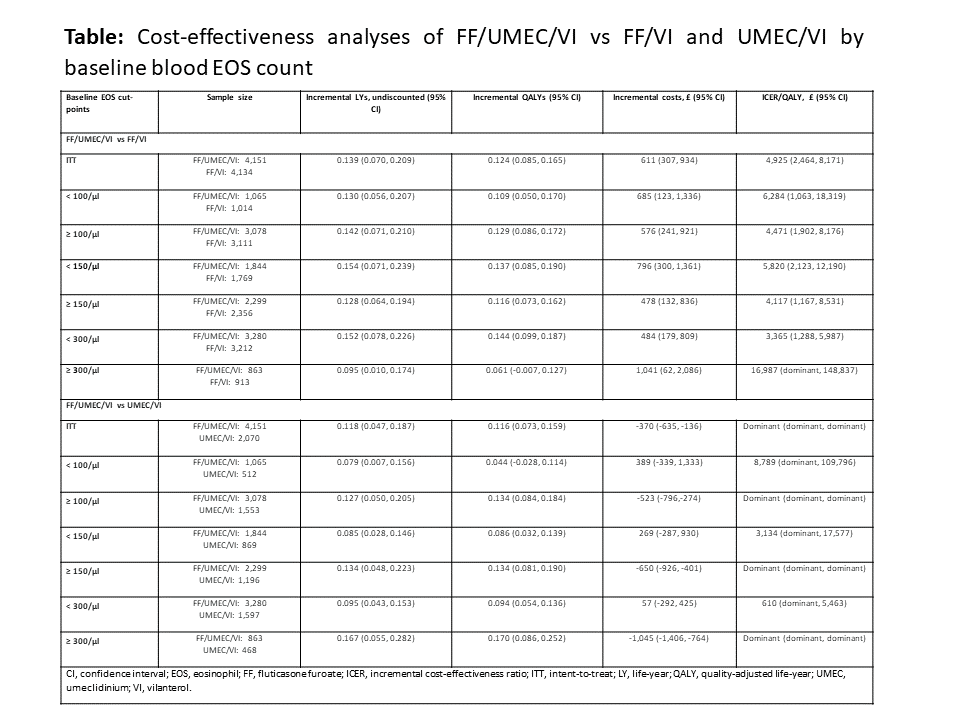
METHODS: Baseline characteristics and treatment effects for subgroups, defined by baseline EOS count (< or ?100, 150, or 300/?L), were identified from IMPACT and used in a probabilistic analysis using the GALAXY model (Briggs, AH. et al. Med Decis Making 207;37:469-80).
RESULTS: Over a life-time horizon, at a willingness-to-pay threshold of £20,000 per quality-adjusted life year (QALY), FF/UMEC/VI was a cost-effective treatment option vs comparators across all EOS subgroups (Table). For FF/UME/VI vs UMEC/VI, incremental QALYs were higher, and costs were lower for subgroups with higher baseline EOS count. Results were consistent across most scenarios with shorter time horizons, alternate discount rates and treatment discontinuation assumptions, and inclusion of patient productivity costs.
CONCLUSIONS: FF/UMEC/VI is expected to be a cost-effective treatment option for COPD patients in the UK, irrespective of baseline blood EOS count.
Funding: GSK 206973