Abstract
Background: Gefapixant was studied in two phase 3 trials in individuals with refractory or unexplained chronic cough (RCC/UCC) for ?1 y and a phase 3b trial in individuals with cough history >8 wk but RCC/UCC for <1 y. Trial comparisons can inform if gefapixant response is influenced by RCC/UCC duration.
Aims and objectives: To compare efficacy/safety of gefapixant after 12 wk of treatment in RCC/UCC for ?1 vs <1 y.
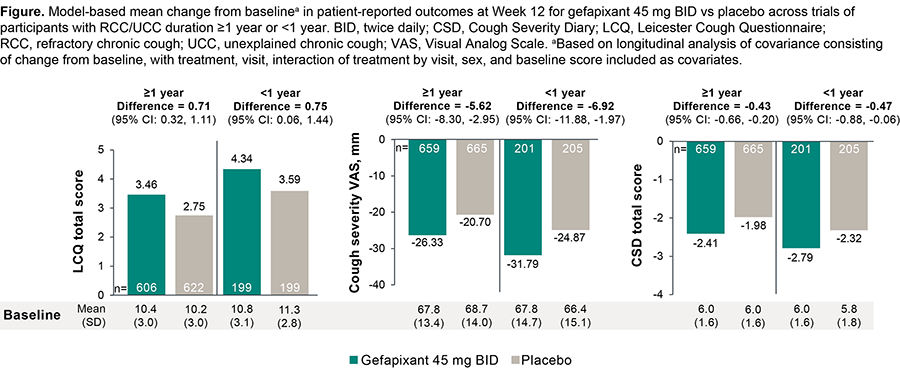
Methods: Participants with RCC/UCC for ?1 y (NCT03449134, NCT03449147; pooled) or <1 y (NCT04193202) who received gefapixant 45 mg BID or placebo (pbo) were analyzed. Change from baseline in patient-reported outcomes (PROs; Leicester Cough Questionnaire [primary endpoint for <1-y trial], cough severity Visual Analog Scale, and Cough Severity Diary) and safety were evaluated at Wk 12.
Results: Other than cough duration (mean [SD]: 11 [9] y vs 7 [3] mo; median [range]: 8 [2-65] y vs 8 [1-12] mo), demographics and baseline cough characteristics were similar across trials (?1 y: gefapixant, n=682; pbo, n=678; <1 y: gefapixant, n=206; pbo, n=209). Gefapixant improved PROs vs pbo with similar efficacy, regardless of RCC/UCC duration (Figure). For gefapixant, adverse event (AE) incidence was 80% (?1 y) and 64% (<1 y); for the taste AEs (?1 y, 64%; <1 y, 54%), >95% were mild or moderate.
Conclusions: Results suggest similar favorable response to gefapixant regardless of RCC/UCC duration.