Abstract
Background
Tezepelumab improved pre-bronchodilator percent predicted FEV1 (pre-BD ppFEV1) versus placebo in patients with severe, uncontrolled asthma in the phase 2b PATHWAY (NCT02054130) and phase 3 NAVIGATOR (NCT03347279) studies.
Objective
This post hoc pooled analysis assessed the effect of tezepelumab on pre-BD ppFEV1 in patients grouped by baseline pre-BD ppFEV1 in PATHWAY and NAVIGATOR.
Methods
Included patients (12?80 years) received tezepelumab 210 mg or placebo subcutaneously every 4 weeks for 52 weeks. Patients had post-BD FEV1 reversibility ?12% and a pre-BD ppFEV1 <80% (<90% for adolescents) before enrolment. Pre-BD FEV1 was assessed by baseline pre-BD ppFEV1 (<80% and ?80%).
Results
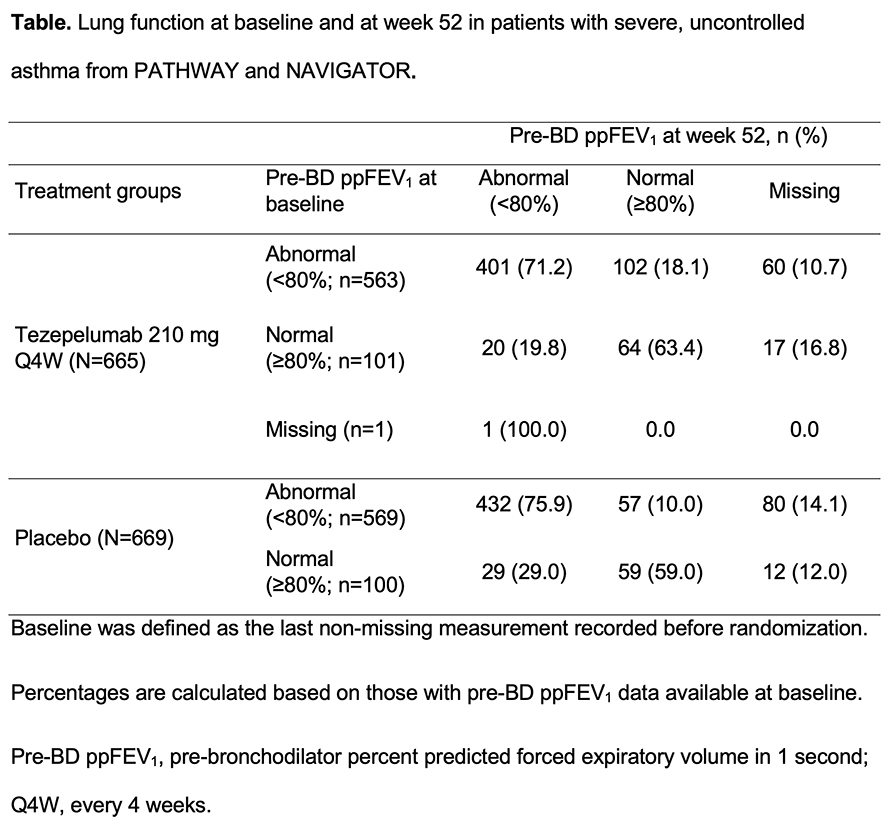
Overall, 665 and 669 patients received tezepelumab or placebo; 563 (84.7%) and 569 (85.1%) had baseline pre-BD ppFEV1 <80%, respectively. Of these, 18.1% and 10.0% had pre-BD ppFEV1 ?80% at week 52 with tezepelumab and placebo (Table). In those with baseline pre-BD ppFEV1 ?80%, fewer tezepelumab than placebo recipients had pre-BD ppFEV1 <80% at week 52 (19.8% vs 29.0%). Tezepelumab improved pre-BD FEV1 at week 52 versus placebo by 0.14 L (95% CI: 0.09, 0.19) and 0.13 L (95% CI: 0.01, 0.24) in patients with baseline pre-BD ppFEV1 <80% and ?80%, respectively.
Conclusion
In patients with severe, uncontrolled asthma and baseline pre-BD ppFEV1 <80%, more tezepelumab than placebo recipients achieved pre-BD ppFEV1 ?80% at week 52.