Abstract
Background
Tezepelumab reduces both blood eosinophil counts (BECs) and fractional exhaled nitric oxide (FeNO) levels versus placebo in patients with severe, uncontrolled asthma. The proportion of patients achieving low type 2 biomarker levels with tezepelumab treatment has not been previously evaluated.
Objective
To assess the proportion of patients who achieved biomarker levels below those associated with an increased risk of asthma-related morbidity (BEC <150 cells/µL or <300 cells/µL; FeNO <25 ppb or <50 ppb) with tezepelumab versus placebo in the phase 3 NAVIGATOR study (NCT03347279).
Methods
NAVIGATOR was a multicentre, randomized, double-blind, placebo-controlled study. Patients (12?80 years old) received tezepelumab 210 mg or placebo subcutaneously every 4 weeks for up to 52 weeks. BECs and FeNO levels were compared at baseline and week 52.
Results
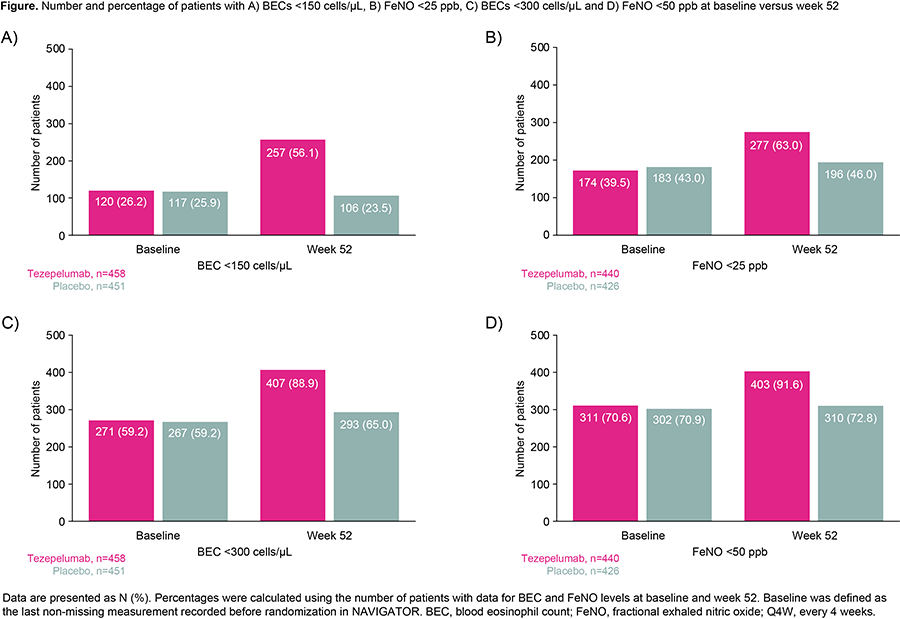
Overall, 528 and 531 patients received tezepelumab and placebo, respectively. At week 52, a greater proportion of tezepelumab recipients achieved BEC <150 cells/µL and <300 cells/µL, and FeNO levels <25 ppb and <50 ppb versus placebo recipients (Figure).
Conclusion
At week 52, most tezepelumab recipients in NAVIGATOR had maintained or reduced their biomarker levels to below those associated with an increased risk of asthma-related morbidity.