Abstract
Background
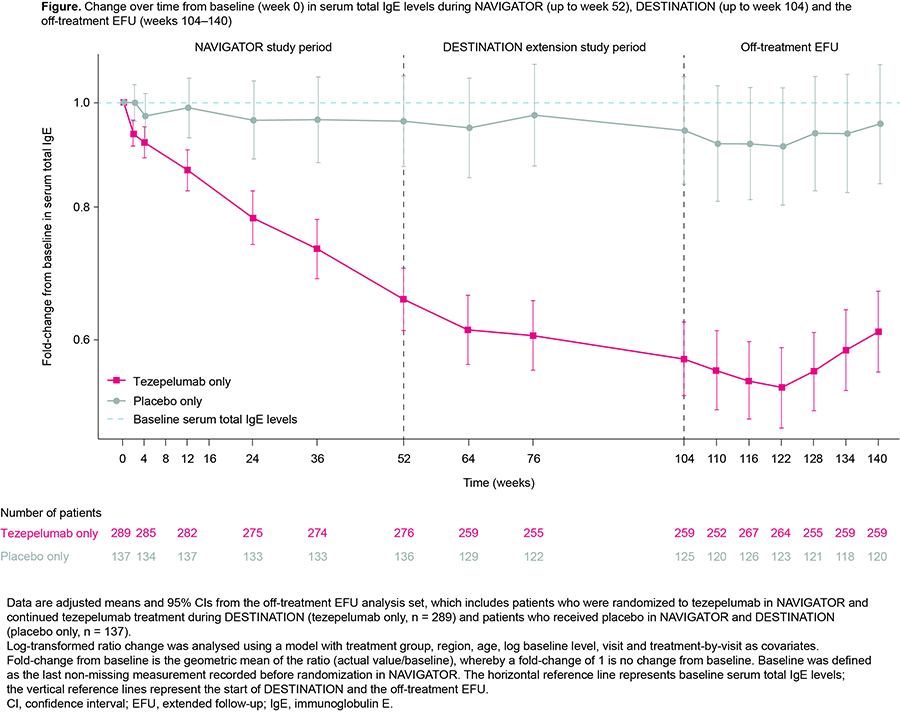
In the phase 3 NAVIGATOR study (NCT03347279), tezepelumab gradually decreased serum total immunoglobulin (Ig)E levels in patients with severe, uncontrolled asthma over 52 weeks. In the phase 3 DESTINATION study (NCT03706079), this reduction continued to end of treatment (EOT; week 104).
Objective
To assess serum total IgE during the off-treatment extended follow-up (EFU) in DESTINATION, following tezepelumab cessation.
Methods
Patients (12?80 years) who completed NAVIGATOR could enrol in DESTINATION, a multicentre, randomized, placebo-controlled, double-blind, extension study. Patients previously randomized to tezepelumab continued treatment (tezepelumab only; 210 mg every 4 weeks). Those previously randomized to placebo were re-randomized 1:1 to placebo (placebo only) or tezepelumab. At EOT, eligible patients could enter a 36-week off-treatment EFU. Change in serum total IgE over time was assessed.
Results
Overall, 569 patients entered the EFU. Mean baseline serum total IgE was 586.18 and 625.19 IU/mL for the tezepelumab only and placebo only subgroups. After tezepelumab cessation, serum total IgE remained low; there was a partial increase from week 122, but levels remained below baseline (Figure).
Conclusion
Serum total IgE levels reductions achieved with tezepelumab during NAVIGATOR and DESTINATION were maintained for 36 weeks after tezepelumab cessation, suggesting a maintained immunological effect.