Abstract
Introduction: There is limited information on how age of asthma onset affects response to therapy. In phase 3 LIBERTY ASTHMA QUEST (NCT02414854), dupilumab, a human monoclonal antibody blocking IL-4/-13, key and central drivers of type 2 inflammation, significantly reduced annualized severe exacerbation rate (AER) and improved lung function in patients with moderate-to-severe asthma. LIBERTY ASTHMA TRAVERSE (NCT02134028) is an open-label extension study evaluating dupilumab long-term safety and efficacy.
Aims and objectives: Evaluate dupilumab efficacy by age of asthma onset in patients with type 2 asthma (blood eosinophils ? 150/?L or fractional exhaled nitric oxide ? 25 ppb) in QUEST and TRAVERSE.
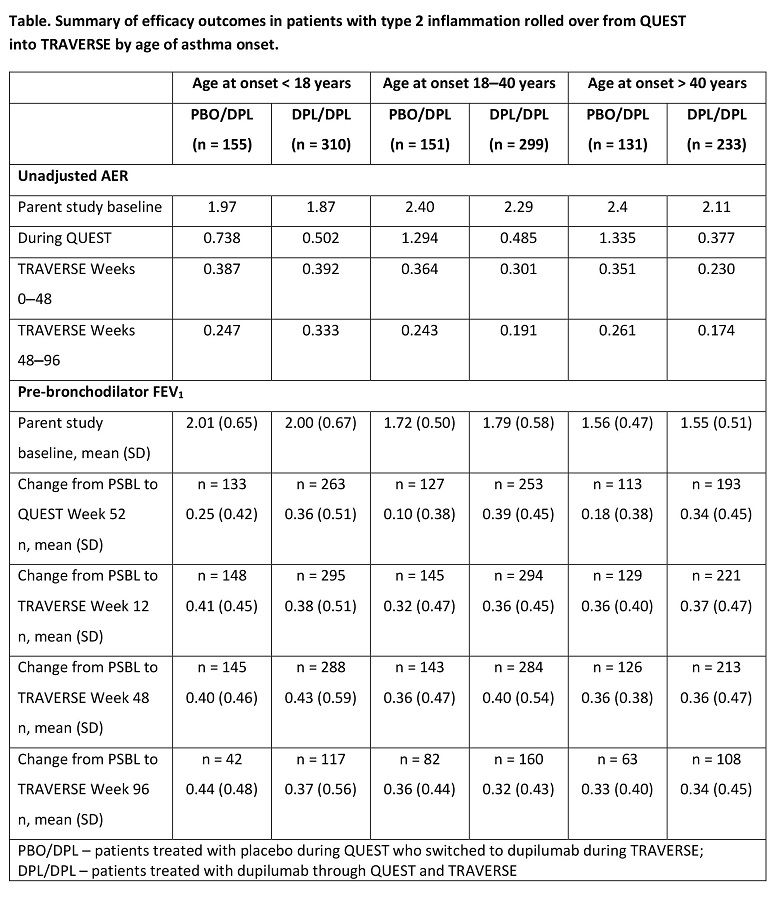
Methods: Patients were stratified by age of asthma onset (< 18, 18?40, > 40 years). Endpoints: AER and change from parent study baseline (PSBL) in pre-bronchodilator FEV1 over QUEST and TRAVERSE.
Results: In patients treated with dupilumab in QUEST and TRAVERSE, AER decreased during QUEST and continued to decrease in TRAVERSE, independently of age of asthma onset (Table). Improvements in FEV1 during QUEST were maintained during TRAVERSE. In patients who received placebo during QUEST, dupilumab significantly improved AER and FEV1 up to Week 96 in all subgroups (Table).
Conclusions: Irrespective of the age of asthma onset, dupilumab reduced AER and improved lung function up to 148 weeks.