Abstract
Rationale: Despite depletion of blood and sputum eosinophils by benralizumab, a subset of patients remain symptomatic (asthma control questionnaire, ACQ-5?1.5).
Objective: To investigate cytokines associated with residual symptoms post-benralizumab.
Method: Sputum was assessed for cytokines (EllaTM,Protein Simple) collected at baseline (V1), after placebo (V3), and 5 injections of benralizumab (V10) in 20 severe asthma patients (11 females, 57±13 years) recruited in a sequential placebo-controlled trial (NCT03470311), previously uncontrolled (ACQ 1.5; sputum eos 3%) on mepolizumab/reslizumab.
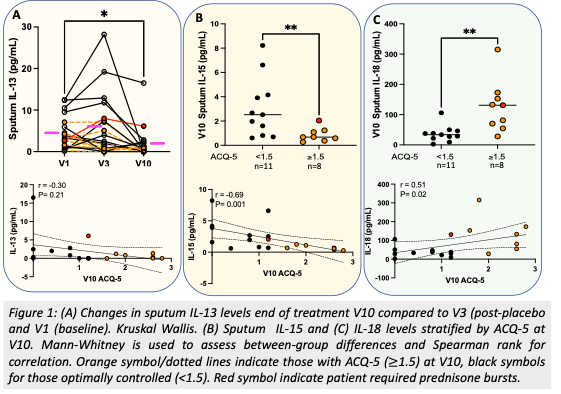
Results: Benralizumab allowed significant decrease in sputum IL-13 (Fig1A), but increases in IL-6 and TNFa compared to V1, irrespective of ACQ-5 and fractional exhaled nitric oxide (FENO). At V10, optimal response, marked by ACQ-5<1.5 was achieved in 11 (55%) patients (mean±SD:0.6±0.5), while n=9 had partial response (PR) (ACQ: 2.2±0.6). Stratified by ACQ at V10 there was no difference in T2 biomarkers: FENO, IL-5/4/13/33 (P>0.05, Mann-Whitney). Increased IL-18 and decreased IL-15 were observed in PR subgroup that further correlated with ACQ-5 (Fig1B-C). In PR group, n=4 with no improvement (ACQ,V10:2.1±0.6;V10-V1:+0.5±6) had CT mucus plugging.
Conclusions: Residual symptoms despite depleted eosinophils post benralizumab may be associated with non-T2 cytokines linked to inflammasome activation (IL-18), reduced NK cell activation (IL-15), and mucus plugging.