Abstract
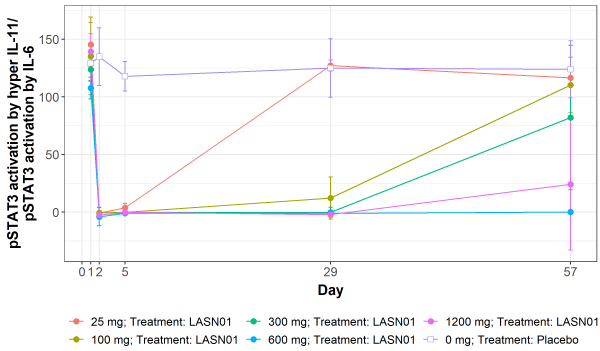
Interleukin-11 (IL-11) is a key effector of fibro-inflammatory disease processes in multiple organs. LASN01 is a highly specific, fully human monoclonal antibody that binds to IL-11R with high affinity and inhibits IL-11 signaling. An integrated Phase 1 trial was conducted to assess safety, pharmacokinetics (PK) and target engagement with single and multiple ascending doses of intravenous LASN01 in healthy volunteers. A total of 58 volunteers were dosed in 5 single dose cohorts (25-1200 mg) and 2 multiple dose cohorts (600-1200 mg). All cohorts were blinded and randomized, 3:1 LASN01 to placebo. An additional study arm is planned to administer multiple doses of LASN01 to patients with pulmonary fibrosis. To date, blinded data from this ongoing study reported 47 adverse events (AEs) which included no serious or severe adverse events. The most common AEs were headaches (10 events, 3 potentially related) and GI discomfort (3 events). The drug PK was dose linear. LASN01 had a half-life of >12 days in patients given a single dose of either 600 or 1200 mg and supports exploring monthly dosing in subsequent studies. A proprietary assay using ex-vivo stimulated whole blood showed >95% inhibition of IL-11R signaling by LASN01 with a single 300 mg dose or 600 mg dose that persisted for 4 and 8 weeks respectively. LASN01 is well tolerated, and robustly inhibits the IL-11 pathway in human volunteers.