Abstract
Introduction
Oral anti-fibrotics attenuate lung function decline in IPF however side effects are common, and often result in discontinuation or dose reduction. Nebulised pirfenidone achieves a 35-fold higher peak epithelial lining fluid concentration and 1/15th of the systemic exposure than standard dose oral pirfenidone, with less frequent side effects and stabilisation of FVC in phase Ib study (West A, et al. Thorax 2023, accepted for publication).
Methods
A phase II open-label extension study of nebulised pirfenidone for progressive fibrosing interstitial lung diseases, including IPF, continues at 22 sites in 6 countries. A total of 72 patients with IPF were recruited; 41 had participated in the phase 1b study (cohort 1) and 31 were newly enrolled (cohort 2). FVC data at 48 weeks was available for 48 patients with IPF between June 2021 and February 2023.
Results
Median treatment duration was 424 and 318 days for cohorts 1 & 2 respectively. The mean±SD change in FVC from baseline at 48 weeks was -165.5±242.6 mls and -151.1±358.9 mls. Previously published clinical trial data of oral pirfenidone in IPF demonstrated a mean change in FVC at 52 weeks of -235 mls (treatment) and -428 mls (placebo) (King Jr TE, et al. NEJM 2014 Sep 18;371(12):1172).
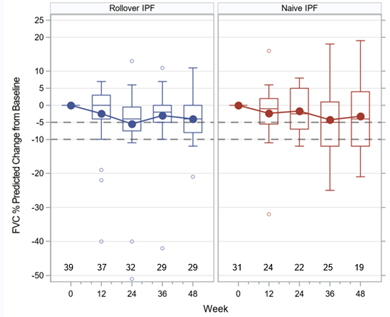
Conclusion
These findings suggest that nebulised pirfenidone solution has the potential to be a better tolerated, effective treatment for patients with IPF and merits further investigation.