Abstract
Background
There is evidence of the rapid action of benralizumab in patients with severe eosinophilic asthma (SEA).
Aims and objectives
To characterize ORBE II study patients with rapid response per ACT and analyze the clinical evolution of these patients after 12 months of benralizumab treatment.
Methods
ORBE II (NTC04648839) is a retrospective multicenter study conducted in SEA patients treated with benralizumab in Spain. In this analysis, rapid responders (RR) were defined as those with an increase in ACT score ?9 or ACT ?24 in the first 120 days after treatment start.
Results
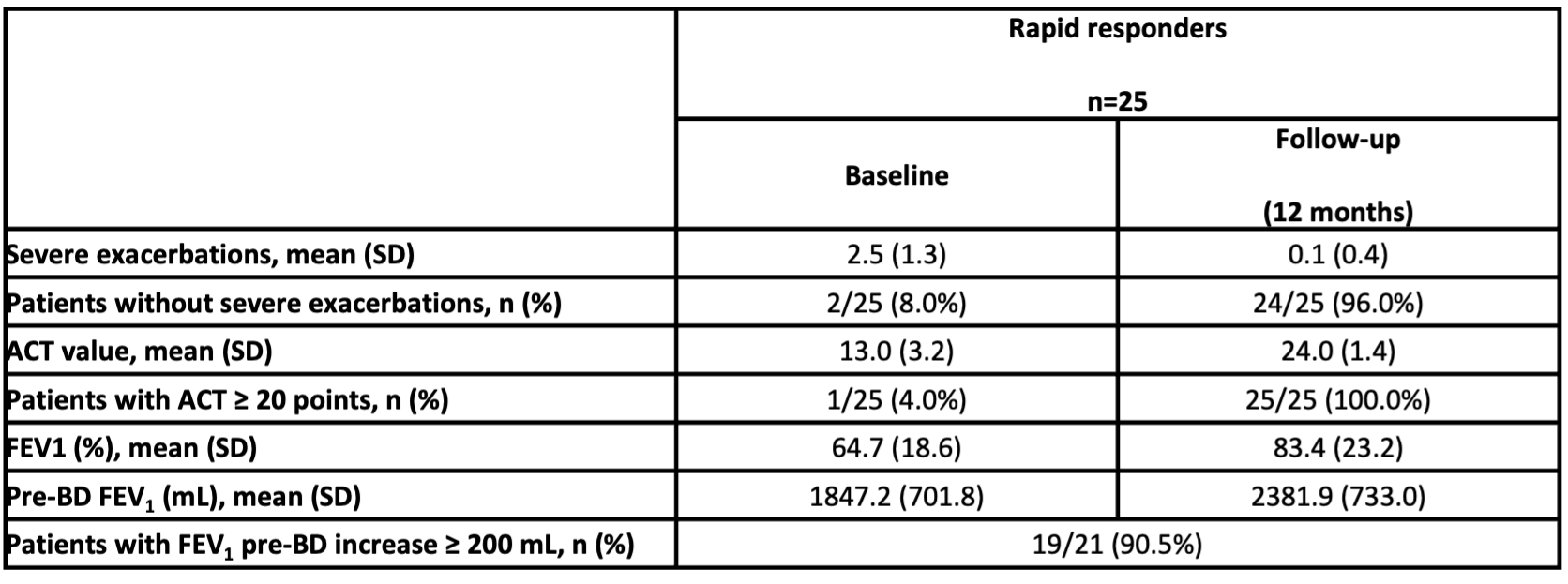
Out of 204 patients, ACT data for the first 120 days were available for 50 patients, of which 25 were identified as RR. This group had a high proportion of non-smokers and presence of nasal polyposis, an elevated baseline eosinophilia, were generally biologic-naïve, and had low baseline ACT (13 points).
After 12 months of treatment with benralizumab, almost all RR eliminated exacerbations, achieved asthma control and improved their pulmonary function.
Conclusions
A substantial proportion of patients could be defined as RR to benralizumab and showed a high level of response after one year of treatment.