Abstract
Background: Dupilumab (DPL) blocks IL-4/-13, key drivers of type 2 inflammation.
Aim: Multicenter, double-blind, phase 3 study (NCT03782532) assessed DPL in patients from China and India with persistent asthma.
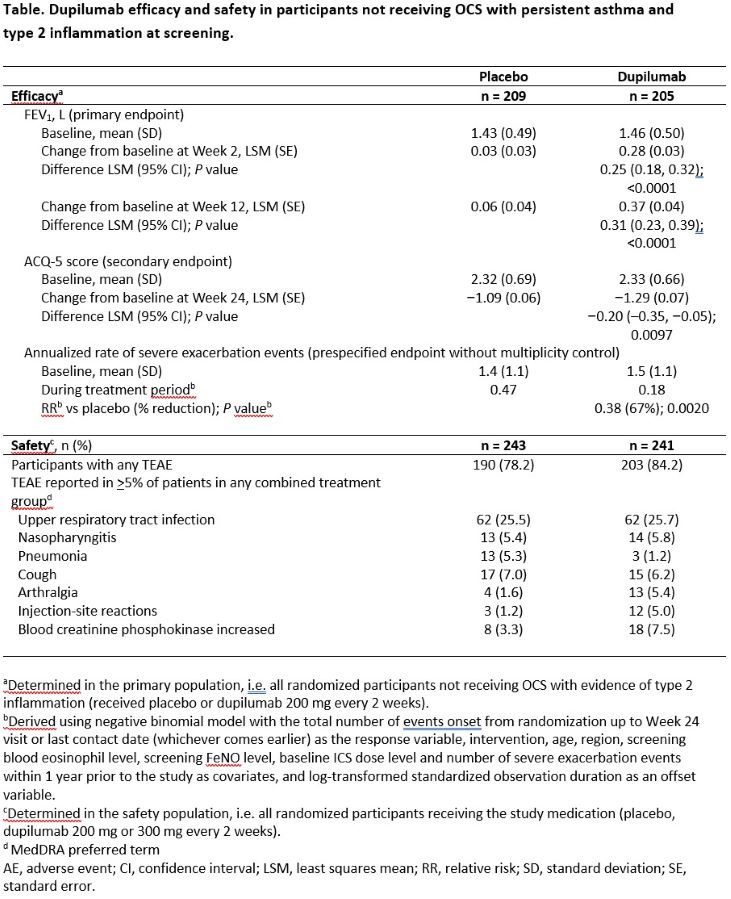
Methods: Patients aged ?12 years with uncontrolled persistent asthma, despite inhaled corticosteroids (CS) +?1 other controller ±oral CS (OCS) randomized 1:1 to DPL (200mg [no OCS maintenance] or 300mg [OCS maintenance] after loading dose) or placebo (PBO) every 2 weeks for 24 weeks. Primary population: participants with type 2 asthma (EOS ?150 cells/?L or FeNO ?25 ppb) and no OCS at screening. Primary endpoint: change from baseline (BL) in pre-bronchodilator (BD) FEV1 at Week 12. Secondary endpoints: change from BL in ACQ-5 score at Week 24; annualized severe exacerbation rate (AER); treatment-emergent adverse events (TEAE).
Results: In the primary population (n=414), DPL significantly increased pre-BD FEV1 at Week 12 vs PBO (least squares mean difference, LSMD [95% CI] 0.31 [0.23,0.39] L; P<0.0001) and reduced ACQ-5 scores at Week 24 (LSMD [95% CI] ?0.20 [?0.35,?0.05] L; P=0.0097). There was a 62% relative reduction in AER during the study with DPL vs PBO (P=0.002). In the safety population (n=484), TEAE were similar with DPL (84.2%) vs PBO (78.2%).
Conclusion: In patients with persistent type 2 asthma and no OCS maintenance, DPL led to rapid, sustained improvement in lung function and asthma control, reduced AER, and was generally well tolerated.