Abstract
Introduction: The use of systemic corticosteroids (SCS), including oral corticosteroids (OCS) is a common therapeutic option in acute asthma and its maintenance management. RAPID (NCT04287621) is a global, prospective registry that aims to characterize patients with asthma initiating dupilumab in real-world clinical practice.
Objective: To report baseline characteristics with focus on SCS use in patients enrolled in RAPID.
Methods: RAPID is enrolling patients aged ?12 years who initiate dupilumab for asthma (primary indication) according to country-specific prescribing information.
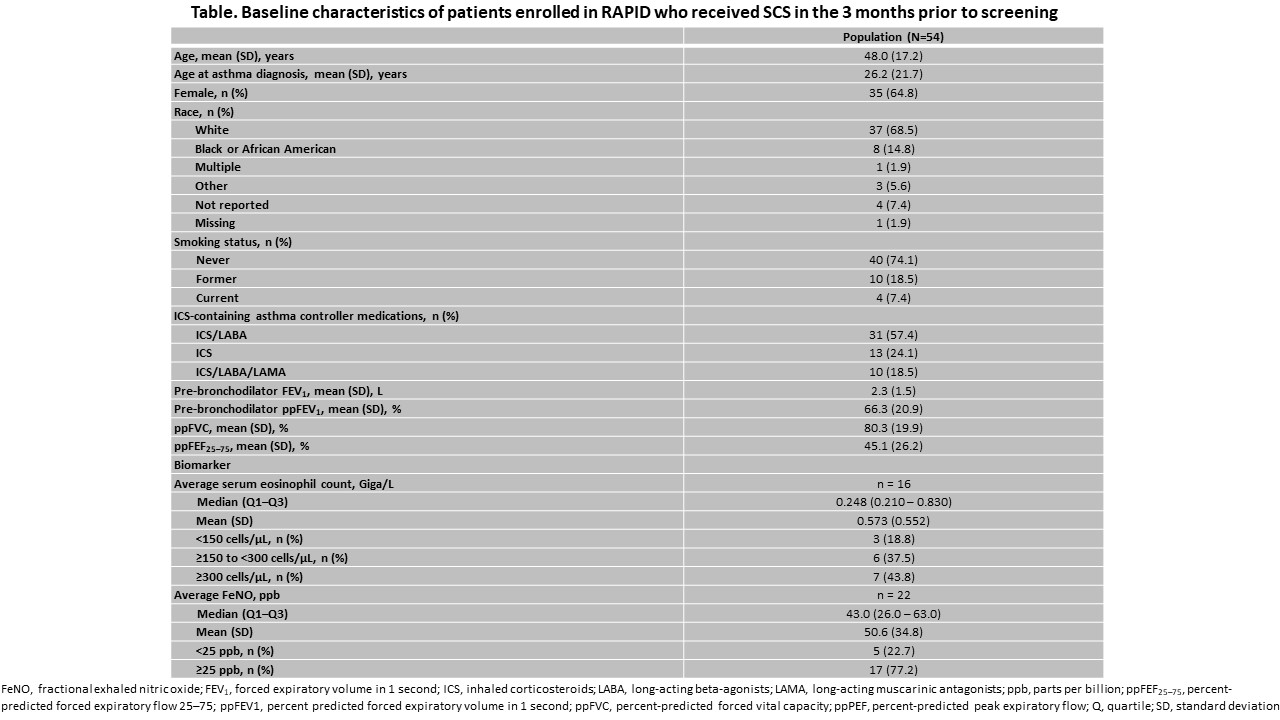
Results: Between Mar 6, 2020 and Oct 28, 2021, 205 patients were enrolled. 54/205 pts (26%) had received SCS in the 3 months prior. OCS treatment was ongoing for 25 patients (48%). All patients were on maintenance inhaled corticosteroids (ICS) (Table). Moreover, 13/16 (81%) patients with baseline blood eosinophil counts available had counts of ? 150 cells/mL and 17/22 (77%) patients with baseline fractional exhaled nitric oxide (FeNO) available had FeNO ? 25 ppb. Other baseline characteristics and inflammatory biomarkers are shown (Table).
Conclusions: In this initial presentation of baseline characteristics of patients from RAPID initiating dupilumab for moderate-to-severe asthma, we show that ? 25% of patients were currently or had recently received SCS, indicating a high unmet need in this population.