Abstract
Background: In the MANDALA study (NCT03769090), as-needed albuterol?budesonide 180/160?g (ABD) significantly reduced severe exacerbation risk by 27% vs albuterol (A) in pts ?12 years with moderate-to-severe asthma on ICS-containing maintenance therapy.
Aim: To explore use patterns of as-needed ABD and A in MANDALA.
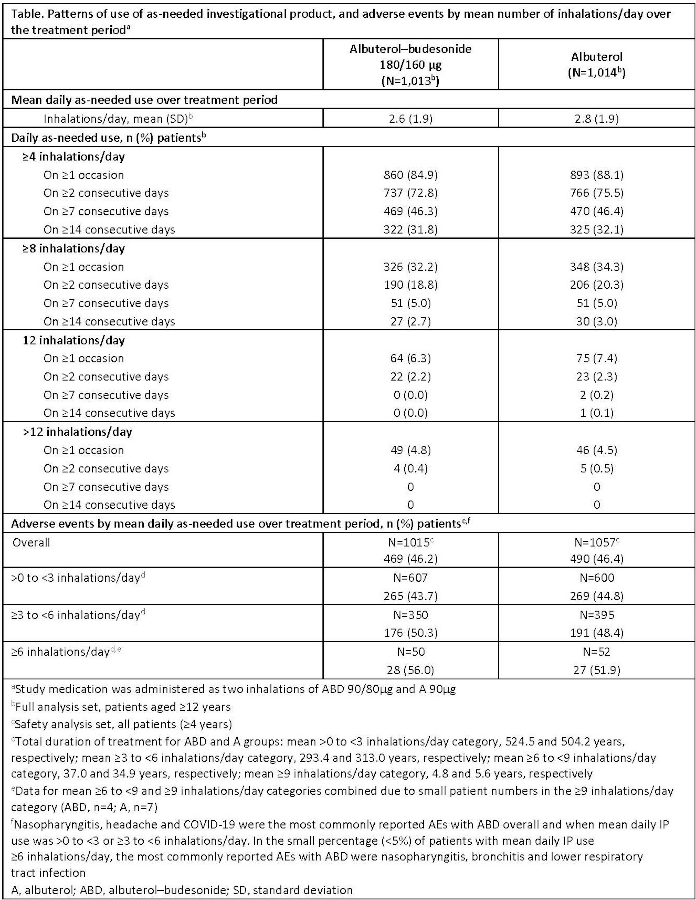
Methods: Patterns of use of investigational product (IP) were evaluated in pts ?12 years randomised to as-needed ABD 180/160?g (n=1013) or A 180?g (n=1014) (administered as 2 inhalations of 90/80?g and 90?g, respectively). IP use was documented in an eDiary.
Results: ABD and A use patterns were similar. Mean % of days with IP use showed pts used 0??2 inhalations on 50?54% of days, ?4 inhalations on 85% of days, and >8 inhalations on <2% of days. In the ABD and A groups, only 5.0% of pts in each group used ?8 inhalations on ?7 consecutive days, 2.2% and 2.3%, respectively, used 12 inhalations (max. per protocol) on ?2 consecutive days, and 0.4% and 0.5%, respectively, used >12 inhalations on ?2 consecutive days (Table). AE frequency was comparable between treatment groups, overall and with increasing mean daily IP use (Table). The small percentage of pts using a mean of ?6 inhalations/day of ABD (<5%) had no clinically important increase in AEs vs A.
Conclusions: Use patterns were similar between treatment groups indicating pts use ABD rescue in the same manner as their SABA. High daily use was uncommon. ABD was well tolerated.