Abstract
Introduction: Benralizumab can be an add-on to high-dose (HD) ICS/LABA in patients (pts) with severe eosinophilic asthma (SEA); data supporting ICS dose reductions (without losing asthma control) are lacking. SHAMAL assessed the ability of benralizumab to permit a progressive reduction from HD ICS/LABA down to anti-inflammatory reliever (AIR) whilst maintaining control in SEA pts who were well-controlled on benralizumab.
Methods: Pts with SEA controlled on benralizumab were randomised to tapering of HD ICS/LABA (budesonide/formoterol; n = 168) or stable dosing (n = 43) in a 3:1 ratio. Pts in the tapering arm who maintained control continued to reduce ICS/LABA dose to AIR only. They then entered a 16-week maintenance period. The primary endpoint was the percentage of pts reducing ICS/LABA dose by the end of reduction period (week 32).
Results: 92% of pts reduced ICS/LABA dose: 61% tapered to ICS/LABA AIR only, 15% to medium-dose, and 17% to low-dose; in 96%, reductions were maintained to week 48 (Figure). The mean [SE] change from baseline in the ACQ-5 at Week 32 was 0.16 [0.04], despite ICS/LABA reductions; 92% of pts in the tapering arm had no exacerbations during the taper period. 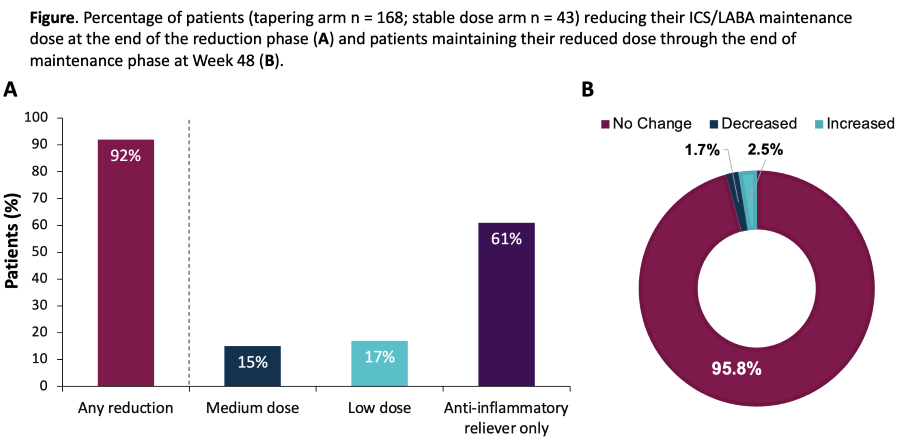
Conclusion: Benralizumab enabled the majority of SEA patients to maintain disease control and remain exacerbation-free despite a reduction in background therapy to AIR only.