Abstract
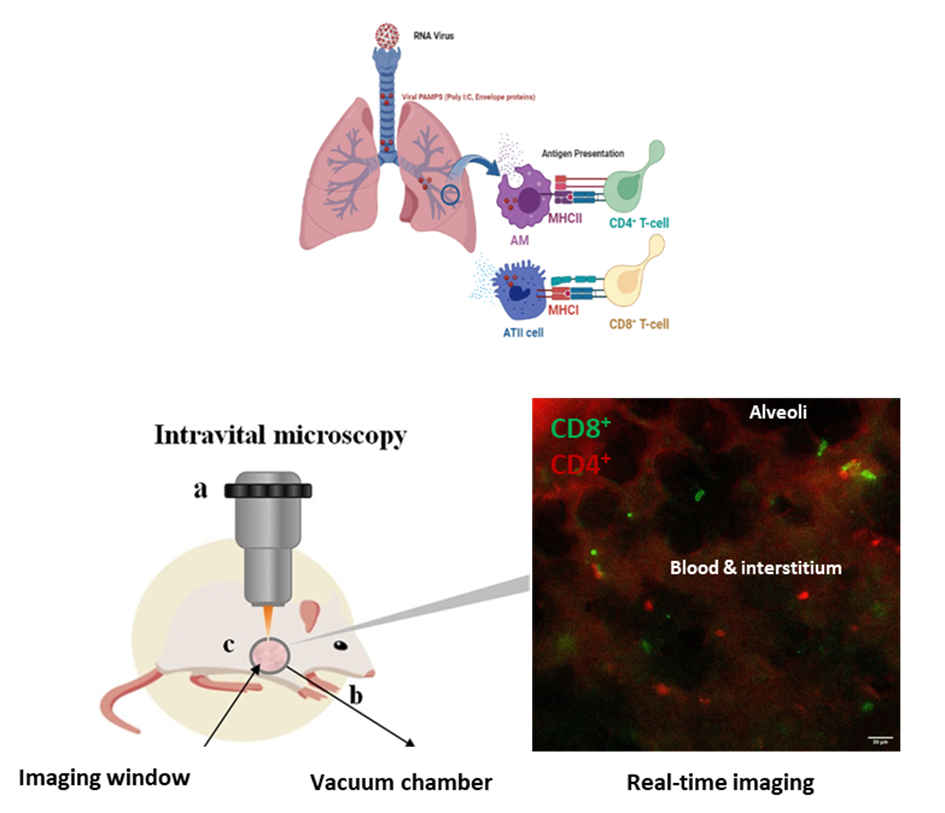
Aims & Methods: Lung intra-vital microscopy (IVM) using fluorescent antibodies or T-cell reporter mice is used to track the real-time spatial and temporal responses of immune cells, specifically CD4+ and CD8+ T-cells in distal live lung, after intra-tracheal instillation with viral PAMPs.
Results: Live microscopy of a co-culture of ATII cells (MLE-12) and alveolar macrophages (MH-S) indicates uptake of dsRNA viral mimetic Poly (I:C) fluorescein mainly by macrophages, which together with ATII cells contribute to the immune response. SARS-Cov2 envelope protein enhanced the transcription of proinflammatory cytokines in MH-S cells and the coculture. IVM showed the CD4+ and CD8+ T-cells, actively patrolling the pulmonary microcirculation in vivo. Immunophenotyping of CD4-Cre X mT/mG reporter mice via FACS shows GFP expression in both CD4+ and CD8+ T-cells, rendering this mouse a pan T-cell reporter. Intra-tracheal instillation of CD4-Cre mice with Poly (I:C) followed by IVM showed significant increase in the number of T-cells recruited to the lung area. Intra-tracheal instillation of C57BL/6J wild type mice with either Poly I:C or SARS-Cov2 Envelope protein, followed by IV injection of fluorescently labelled CD4+ and CD8+ antibodies, showed significant changes in mean speed and net displacement of T-cells
Conclusion: Monitoring the dynamics of lung immune cells can help unveil targets for therapy.