Abstract
Rationale: The airway epithelium is altered in asthma, characterized by goblet cells hyperplasia, loss of ciliated cells and increases in basal cells. It is yet unclear whether these changes are a consequence of the disease process, or whether the cells are inherently different. Here we aim to explore differentiation in air-liquid interface (ALI) and 3D organoid cultures of primary bronchial epithelial cells (PBECs) from patients with asthma and matched controls.
Methods:
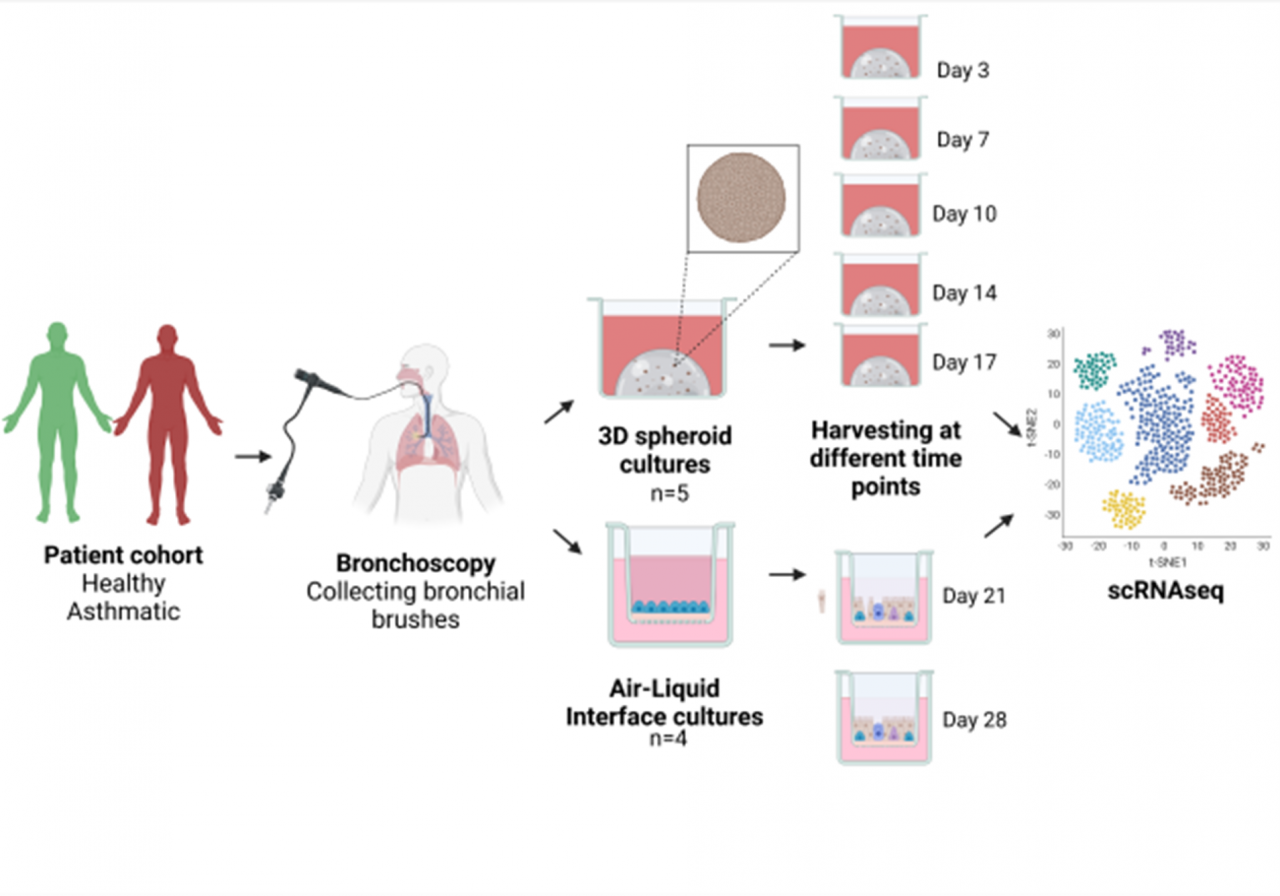
Results: The scRNAseq data show the presence of basal and secretory cells in both types of culture models and at all time points. PBECs grown in spheroid cultures show an increase in the proportion of secretory cells over basal cells in time. However, no ciliated cell differentiation is observed, contrary to the PBECs grown in ALI cultures. Directed single-cell fate mapping reveals two differentiation trajectories from suprabasal cells to secretory cells, with PBECs derived from asthma patients being more prone to differentiate towards squamous-like cells. Gene ontology analysis of the genes driving the differentiation towards club cells suggest higher metabolic activity in the healthy PBECs.
Conclusion: Taken together, our results suggest that the differentiation process of the airway epithelium is altered in asthma, with cell fate decisions being inherently different from PBECs derived from healthy subjects.