Abstract
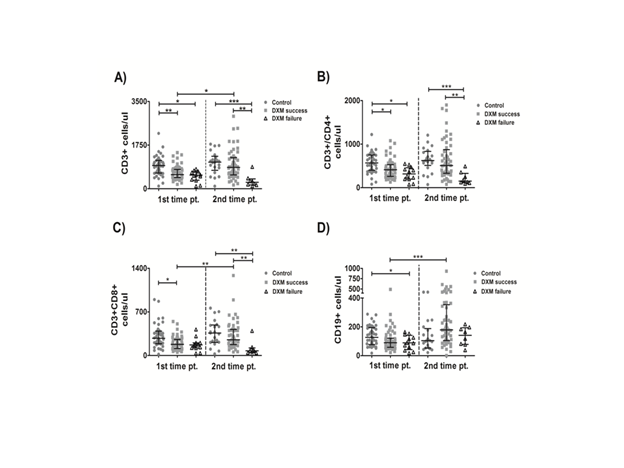
Rationale: Changes of anti-SARS-CoV-2 defense immune-subsets in patients treated with dexamethasone (DXM) for severe COVID-19 and their relation to disease outcomes are poorly understood. Methods: We prospectively evaluated peripheral-blood lymphocyte subsets in 110 hospitalized patients with COVID-19 (who received treatment with corticosteroids or not) and we investigated possible links with final outcomes. The first sample was obtained at enrollment (1st time point- tpA) and the second one (2nd time point-tpB) was obtained 7-10 days later. Total lymphocyte, B-lymphocyte, CD4+, CD8+ Τ-Lympocyte and Natural Killer (NK) cell counts were determined by flow cytometry. Results: The patients in the DXM-failure group (intubation/death) exhibited fewer total CD3+ cells, CD4+, CD8+ T- and B-lymphocytes compared to the control group (no DXM treatment) and/or DXM-success group (hospital discharged) at tpA. DXM-failure group had fewer total CD3+ cells, CD4+ and CD8+ T-lymphocytes compared to the control group and/or DXM-success at tpB-Figure 1. The number of NK (cells/ul) in the DXM-failure group was significantly decreased over time: median (25-75 interquartile range-IQR) tpA= 133.5 (68.75-216.3), tpB= 78 (28.25-101), p<0.05 and it was significantly decreased at the time of treatment completion (tpB) compared to the control group: median (IQR) 78 (28.25-101) vs 160 (113.5-227), p<0.001.
Fig. 1: Lymphocyte kinetics. *p<0.05,**p<0.01,***p<0.001.