Abstract
Despite the advantages of microfluidics over static culture, simulating elements of the tissue?s native 3D environment and providing precise control of experimental design, engagement with these models remains limited due to the high input costs of commercial systems or of independent construction. We developed a large-scale airway-on-chip model with a uniquely accessible sealing procedure that provides a platform that enables sampling at comparable volumes to traditional culture models with the advantages of culture under flow. The model consists of polydimethylsiloxane (PDMS) with a polyethylene (PET) membrane cold-cured into place under vacuum. We show collection of sufficient quantities of RNA for qPCR/RNA sequencing and protein for cytokine detection. Further, we observed epithelial cell-cell contact formation and differentiation into mucus producing cells at the air-liquid interface (ALI) under biological flow rates, as assessed by MUC5AC production. Finally, we show that the device enables co-culture with lung fibroblasts. Collectively, our ?Macrofluidic? airway-on-chip model provides a readily reproducible, adjustable, and cost-effective large-scale fluidic 3D lung cell culture platform for investigating biological and pathological processes of the respiratory system. 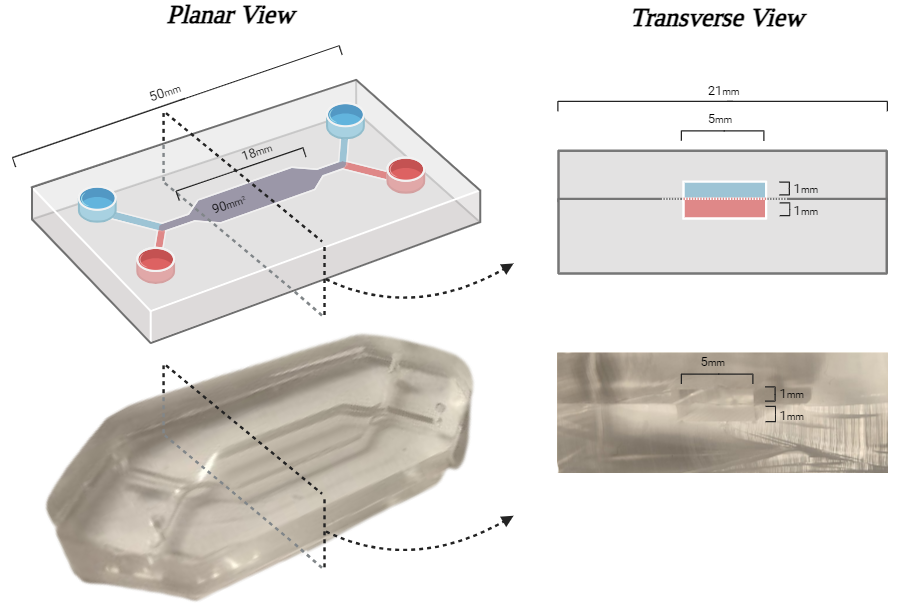
Figure 1: Design of the ?Macrofluidic?.
Left: Airway-on-chip lengths with the 90mm2 cell culture membrane and inlets for air (blue) and medium (red).
Right: Transverse view of devices widths and heights.
Created with Biorender.com