Abstract
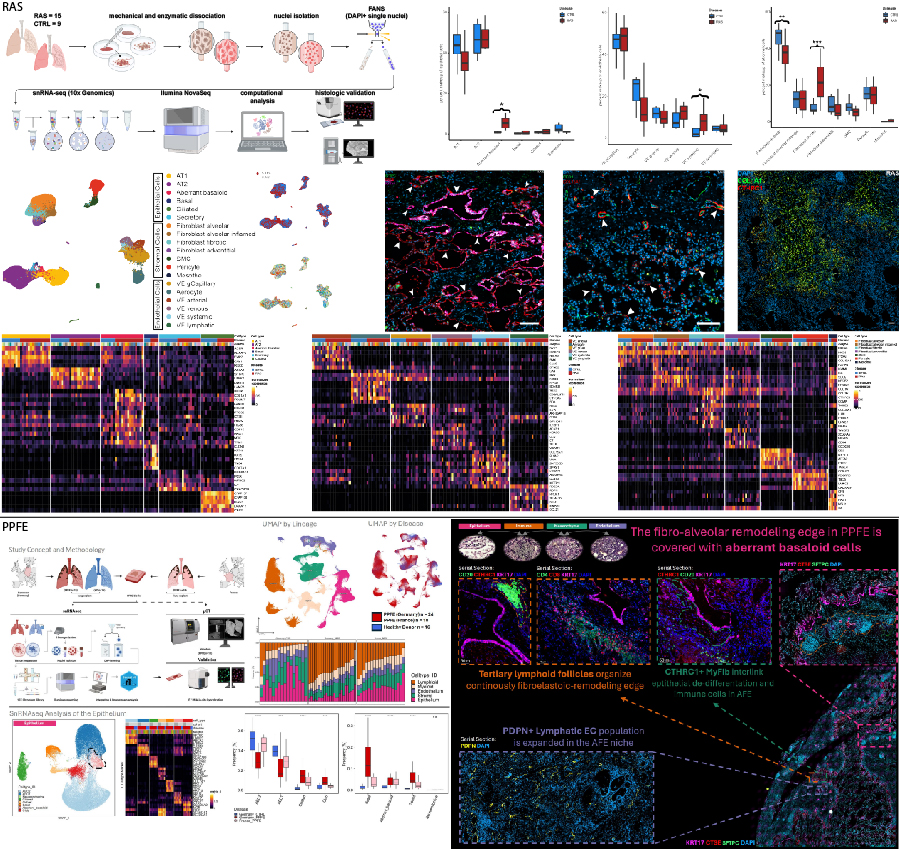
Background: Restrictive allograft syndrome (RAS) and pleuroparenchymal fibroelastosis (PPFE) are progressive fibrotic lung diseases characterized by alveolar fibroelastosis (AFE). Despite poor prognosis, effective treatments remain unavailable, and their pathophysiologies are elusive.
Aim: To uncover common cellularly resolved transcriptomic and histologic features of human RAS and PPFE lungs to identify novel therapeutic targets.
Methods: Peripheral lung tissues from 15 RAS, 32 PPFE, and 16 control lungs were analyzed using snRNA-seq. Results were validated and extended using e.g., immunofluorescence and RNA in situ hybridization.
Results: Our data revealed a shared fibrotic niche in RAS and PPFE at the edge of AFE remodeling. Key findings are: (A) aberrant basaloid cells, overlaying AFE lesions; (B) expansion of CTHRC1+ fibrotic fibroblasts within AFE areas; (C) partial replacement of the physiologic PRX+ alveolar endothelium by ectopic COL15A1+ endothelial cells; and (D) elevated T- and B-cell fractions in PPFE, suggesting immune-mediated repair failure.
Conclusions: RAS and PPFE depict cellular hallmarks of fibrotic tissue organization first described in IPF. Remarkably, our data suggests an underlying immune-mediated process in contrast to the pathogenesis of IPF. These shared cell types, essential to fibrogenesis, might be prime therapeutic targets.